



Find all of your laboratory and workplace safety supplies at Safety Emporium!
 Cytotoxin |
 Glossary Index |
 Density |
| MSDS Topics |
Free Sites | FAQ's | Regulations | Glossary | Software | Suppliers |
| Books | Forum | Poll | Fun stuff | Quiz | Store | |
| Understand your MSDS with the MS-Demystifier | Search ALL our MSDS info | |||||
Just as a piece of food (which is really just a collection of many chemicals) can spoil or rot (see biodegradable), so can certain chemical substances or mixtures.
For example, when glucose (a form of sugar) is burned in air, it decomposes into carbon dioxide and water. This reaction is also an example of an exothermic combustion reaction:
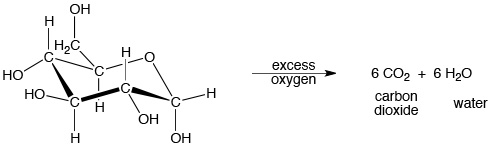
See this discussion of thermodynamics for more information about the energy involved in this and other reactions.
Living organisms exploit decomposition reactions in order to live. For example, the digestion of food is an exothermic process, which provides organisms with heat and chemical energy while converting the food to simpler substances that are then used as building blocks within the body or are discarded as waste.
As we live in a dynamic world, many of the everyday objects around us are thermodynamically unstable. Provided the conditions are right, they will decompose all by themselves or with a little help by Nature. Examples include bread, nitroglycerin, lawn clippings and more. Some of these processes generate enough heat on their own to undergo spontaneous combustion!
Certain classes of chemicals such as peroxides, pyrophoric and water-reactive materials can decompose quite readily, releasing so much heat that they can explode or combust. These highly-reactive materials pose serious hazards and should only be handled by experts.
The OSHA Hazard Communication Standard (HCS) requires the decomposition temperature to be reported in Section 9 of the Safety Data Sheet and any hazardous decomposition products to be reported in Section 10.
Decomposition can have many effects, each of which is potentially hazardous:
See also: HMIS, incompatible chemicals, peroxide, polymerization.
Additional definitions from Google and OneLook.
Entry last updated: Saturday, July 9, 2022. This page is copyright 2000-2024 by ILPI. Unauthorized duplication or posting on other web sites is expressly prohibited. Send suggestions, comments, and new entry desires (include the URL if applicable) to us by email.
Disclaimer: The information contained herein is believed to be true and accurate, however ILPI makes no guarantees concerning the veracity of any statement. Use of any information on this page is at the reader's own risk. ILPI strongly encourages the reader to consult the appropriate local, state and federal agencies concerning the matters discussed herein.